?
Lindane is an organochlorine insecticide, also known as gamma-hexachlorocyclohexane (HCH) and benzene hexachloride (BHC). It has an LD50 of 88 mg/kg, and is a suspected carcinogen. Its method of action is via interfering with the GABAa receptor/Cl- channel. It has been used in agriculture and in pharmaceutical products for the treatment of headlice and scabies.
 Bans
BansLindane is volatile with roughly 90% entering the atmosphere and ultimately being deposited in rain. In 1992 5.5 mg/L of lindane was detected in rain in Oxfordshire.
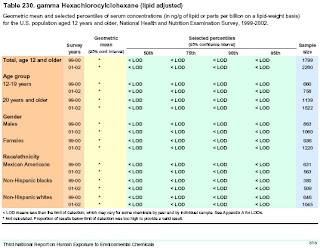
Environmental Contamination
According to Alliant Pharmaceuticals, the U.S. distributor of lindane shampoo and lotion:
Neurologic Toxicity Seizures and deaths have been reported following Lindane Shampoo use with repeat or prolonged application, but also in rare cases following a single application according to directions. Lindane Shampoo should be used with caution in infants, children, the elderly, and individuals with other skin conditions (eg, atopic dermatis, psoriasis) and those who weigh < 110 lbs (50 kg) as they may be at risk of serious neurotoxicity.
Lindane is contraindicated for use in neonates and should be used with extreme caution in children and in individuals weighing less than 50 kg (110 lbs). Among adverse event reports in which the outcome was serious (resulted in hospitalization, disability or death), the very young and the elderly appeared to be more susceptible to Lindane's adverse effects and had worse outcomes.
Animal studies have demonstrated that younger animals are more susceptible to the neurologic side effects seen with Lindane use. In addition, smaller children have a larger body surface to volume ratio that may result in proportionately larger risk of systemic exposure. For this reason, Lindane has long been contraindicated for use in neonates. It is not known whether the developing nervous system of children also increases their susceptibility to neurologic toxicity.
Patients who have conditions, such as HIV infection, or take certain medications that may lower the seizure threshold should be prescribed Lindane with caution. They may be at greater risk for serious adverse events. The new Lindane label lists examples of some of these conditions and medications. The label also highlights special precautions for use of Lindane in women who are breastfeeding infants.
There are case reports of neurologic adverse events in nursing home patients treated with Lindane. Factors that may have increased their susceptibility to these adverse events include concomitant medications, underlying medical conditions, and advanced age. Special consideration should be given prior to treating this population with Lindane, even if they are greater than 50 kg.
Health Effects
In the US, Alliant Pharmaceuticals It has recently been banned for the treatment of head lice in California. In the United States, the Food and Drug Administration requires these products be labeled with prominent warnings about possible neurotoxicity, particularly in young patients.
No comments:
Post a Comment